Oxidation Reduction Potential (ORP) measures the net voltage potential of excess oxidizers or reducers in a liquid. As it is a less common measurement, it can often be misunderstood. It is also referred to as "Redox" since the sensor measures redox reactions. To understand this, it is helpful to grasp the chemistry of these particles.
An oxidizer is a molecule with an excess of electrons, giving it a negative charge, while a reducer is a molecule with a deficit of electrons, resulting in a positive charge.

Image Credit: Hamilton Process Analytics
Any aqueous solution with excess oxidizers or reducers will exhibit a voltage potential. Excess reducers give a net negative charge, while excess oxidizers give a net positive charge, with a neutral liquid having no charge.
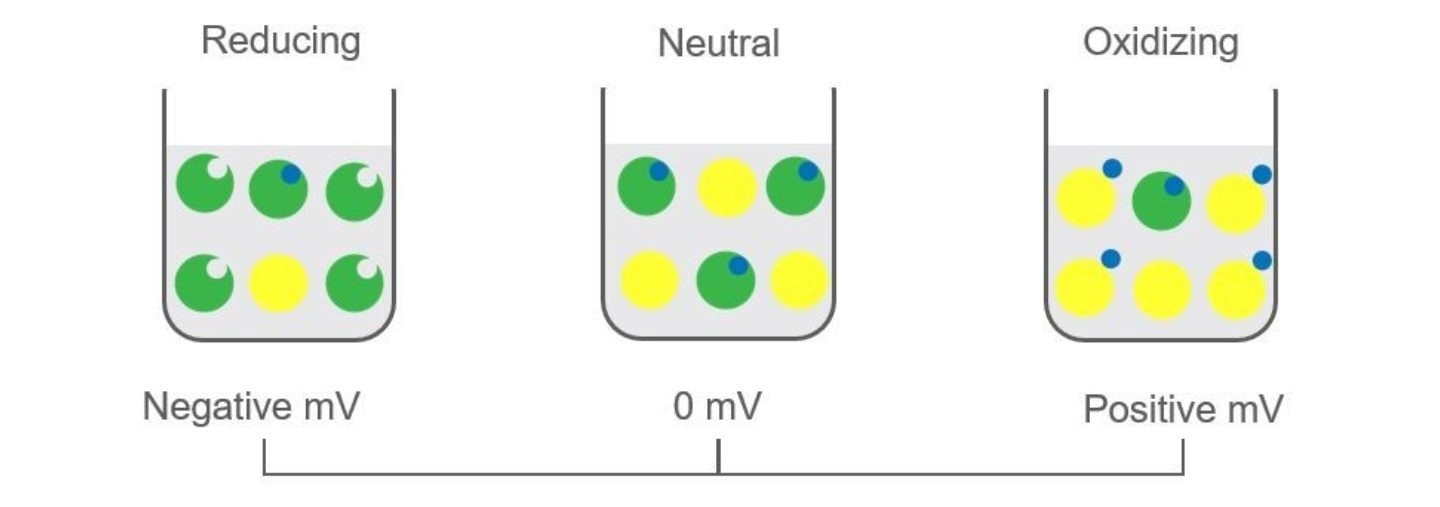
Image Credit: Hamilton Process Analytics
When these molecules come into contact, they engage in a redox reaction involving the transfer of electrons.
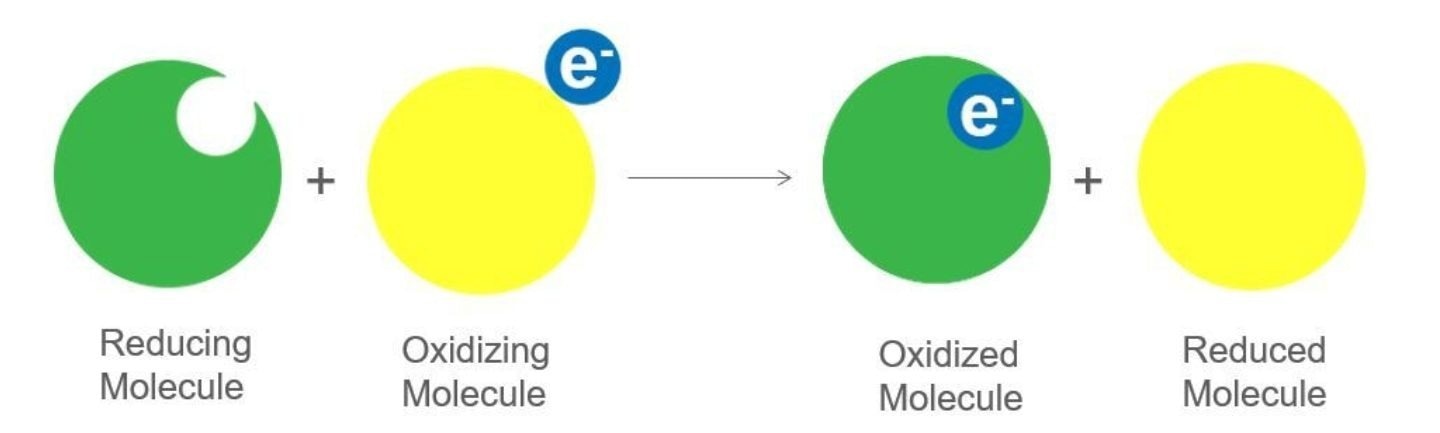
Image Credit: Hamilton Process Analytics
A common example is combustion, such as gasoline burning. Gasoline, primarily composed of octane, combines with oxygen (an oxidizer) under sufficient heat, leading to a redox reaction, as shown by the equation below.
2C8H18 + 25O2 → 16CO2 + 18 H2O
This reaction converts octane and oxygen into carbon dioxide and water vapor. This is why water condensation can be seen in a car's exhaust pipe.
How Do ORP Sensors Work?
The structure of an ORP sensor closely resembles that of a pH sensor, both having a reference electrode and a measurement electrode. The reference electrode is an Ag/AgCl element in a KCl electrolyte.
This reference electrode generates a stable voltage potential of around 220 mV and features a porous liquid junction (sometimes referred to as a diaphragm), which allows the electrolyte to interact with the liquid medium, completing the sensor's voltage circuit.
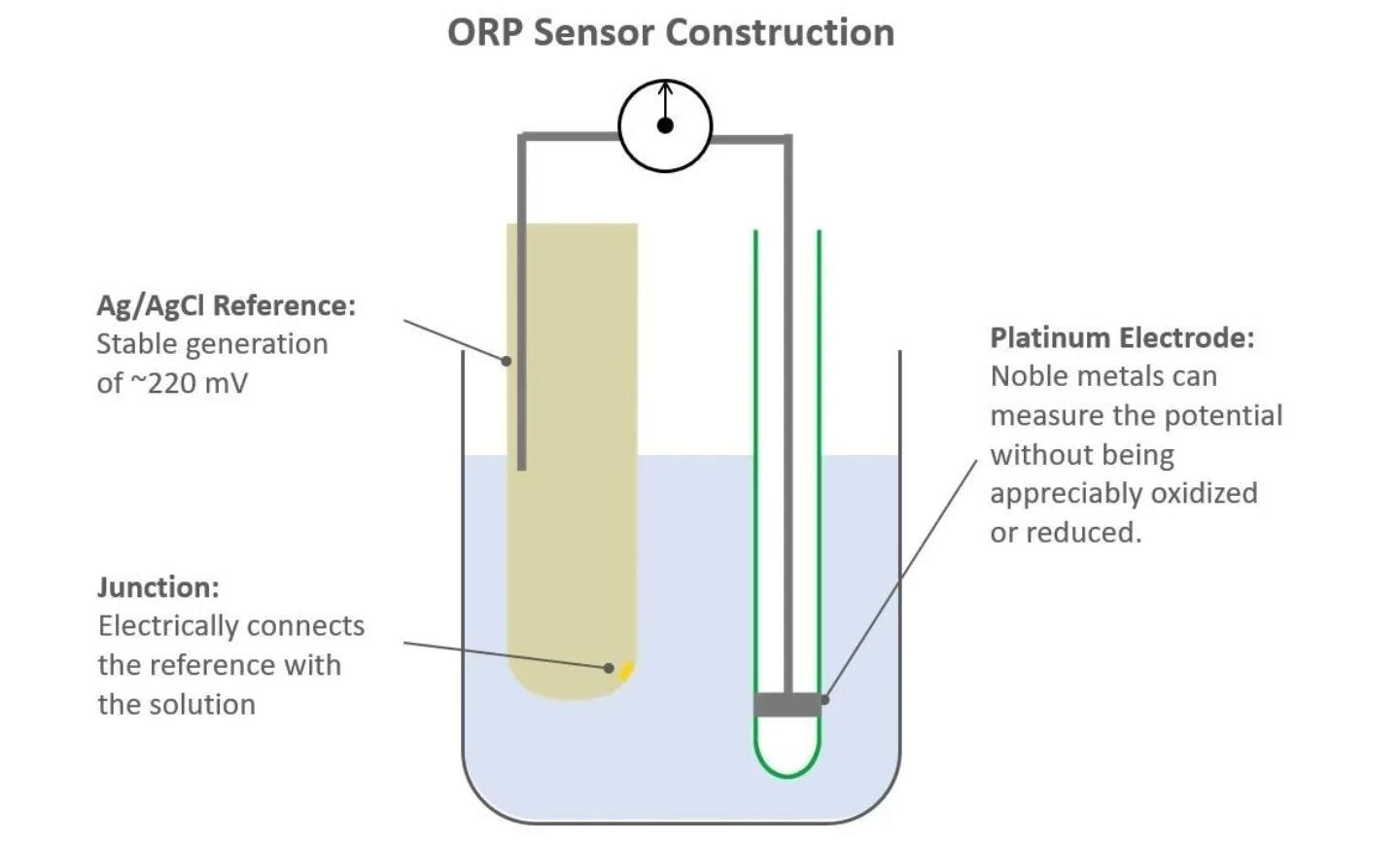
Image Credit: Hamilton Process Analytics
The measurement electrode sets ORP sensors apart from pH sensors. ORP sensors lack the specialized glass membrane seen in pH sensors. Instead, the measurement electrode of an ORP sensor has a metal band or wire in contact with the process liquid.
The metal contact point completes the voltage circuit of the sensor, which also involves the reference electrode and the liquid junction. The metal itself does not generate any potential, but alterations in the concentration of oxidizers or reducers can affect the overall circuit's voltage potential.
Usually, a noble metal like platinum or gold is employed for the measurement electrode to safeguard it from damage caused by the redox reaction. All Hamilton ORP sensors utilize platinum for their measurement electrode.
It is crucial to keep in mind that the reference electrode produces 220 mV. This means that even in a completely neutral solution with no voltage potential, the ORP sensor would still produce 220 mV. To ensure accurate measurement, this voltage is subtracted by the pH transmitter electronics during calibration.
What is the ORP Unit of Measure?
The ORP sensor is not specific to a single oxidizing or reducing molecule, so its output is a raw millivolt value without any other unit of measure.
An increase in voltage indicates a higher concentration of one type of molecule or the other. Therefore, ORP is often employed as a control parameter for redox reactions. For instance, chlorine, a potent oxidizer used for disinfection, affects ORP.
When liquid bleach is added, the ORP value immediately rises. Over time, as chlorine interacts with biological organisms and other molecules, the ORP value decreases.
An ORP setpoint is utilized to manage chlorine addition and optimize disinfection. While ORP does not directly measure chlorine, its measurement serves as a reliable indicator of the reaction's strength.
What Effect Does Temperature Have on ORP?
Temperature directly influences the ORP value of a solution. An increase in temperature accelerates the dissociation of molecules in the liquid, raising the ORP value and the rate of redox reactions.
Unlike pH, where temperature affects the glass membrane potential and requires compensation, the metal measurement electrode does not yield any output.
Consequently, ORP is not temperature-compensated during measurement. However, temperature is considered during calibration. The value of an ORP calibration solution at various temperatures can be predicted and compensated for as part of the calibration process to enhance accuracy.
What Effect Does pH Have on ORP?
pH measures the hydrogen ion concentration in an aqueous solution. Given that positively or negatively charged particles influence ORP, changes in pH directly affect the ORP value.
While pH and ORP tend to change together, their rate of change may not precisely correlate due to the influence of the redox reaction. Take chlorine, for example, mentioned earlier. At pH values below 1.9, chlorine exists as Cl2 in water.
As pH increases, chlorine reacts with water to form hypochlorous acid (HOCl), leading to a decrease in the ORP value, as represented by the formula below.
Cl2 + H2O → HOCl + HCl
At pH levels of 7.3 and above, hypochlorous acid (HOCl) transforms into hypochlorite (OCl-), causing the ORP value to further decline.
HOCl → OCl- + H+
The transitions from Cl2 (a strong oxidizer) to HOCl (a mild oxidizer) to OCl- (a weak oxidizer) impact the effectiveness of chlorine as a disinfectant. Consequently, pH and ORP are jointly employed to control the process.

This information has been sourced, reviewed and adapted from materials provided by Hamilton Process Analytics.
For more information on this source, please visit Hamilton Process Analytics.