Nov 24 2015
Researchers have devised a way to watch the details of your neurons at work.
Every second of every day, the 100 billion neurons in your brain are capable of firing off a burst of electricity called an action potential up to 100 times per second. For neurologists trying to study how this overwhelming amount of activity across an entire brain translates into specific thoughts and behaviors, the task can be…well…overwhelming.
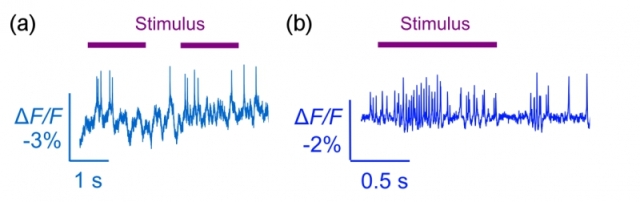 (a) Optically detected spikes from an awake behaving mouse driven by visual stimulus. (b) Optically detected spikes from an awake behaving fly drive by odor stimulus.
(a) Optically detected spikes from an awake behaving mouse driven by visual stimulus. (b) Optically detected spikes from an awake behaving fly drive by odor stimulus.
That’s because existing techniques for monitoring neurons are too slow or have too narrow a scope to generate a holistic view. But in a new study, researchers at Stanford University and Duke University reveal a technique for watching the brain’s neurons in action with a temporal resolution of about 0.2 milliseconds—a speed that is just fast enough to capture the action potentials in mammalian brains.
The paper appeared online in Science on November 19, 2015.
“We set out to combine a protein that can quickly sense neural voltage potentials with another protein that can amplify its signal output,” said Yiyang Gong, assistant professor of biomedical engineering at Duke and first author on the paper. “The resulting increase in sensor speed matches what is needed to read out electrical spikes in the brains of live animals.”
Then working as a postdoc in the laboratory of Mark Schnitzer, associate professor of biological sciences and applied physics at Stanford, and an investigator of the Howard Hughes Medical Institute, Gong and his colleagues sought out a voltage sensor fast enough to keep up with neurons. After several trials, the group landed on one found in algae, and engineered a version that is both sensitive to voltage activity and responds to the activity very quickly.
The amount of light it put out, however, wasn’t bright enough to be useful in experiments. It needed an amplifier.
To meet this engineering challenge, Gong fused the newly engineered voltage sensor to the brightest fluorescing protein available at the time. He linked the two close enough to interact optically without slowing the system down.
“When the voltage sensing component we engineered detects a voltage potential, it absorbs more light,” explained Gong. “And by absorbing more of the bright fluorescent protein’s light, the overall fluorescence of the system dims in response to a neuron firing.”
The new sensor was delivered to the brains of mice using a virus and incorporated into fruit flies through genetic modification. In both cases, the researchers were able to express the protein in the neurons they chose and observe their voltage activity. They were also able to read voltage movements in different sub-compartments of individual neurons, which is very difficult to do with other techniques.
“Being able to read voltage spikes directly from the brain and also see their specific timing is very helpful in determining how brain activity drives animal behavior,” said Gong. “Our hope is that the community will explore those types of questions in more detail using this particular sensor. Already I’ve received multiple emails from groups interested in trying the technique in their own labs.”