Apr 22 2016
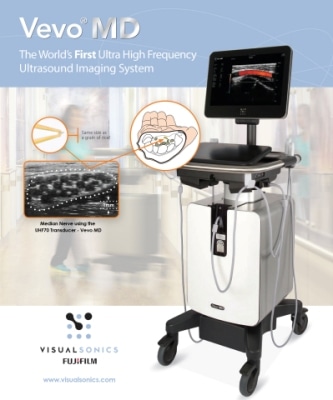
FUJIFILM VisualSonics Inc., a world leader in ultra high frequency ultrasound imaging systems and subsidiary of FUJIFILM SonoSite, Inc., today announced 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Vevo® MD, the world’s first Ultra High Frequency (UHF) clinical ultrasound system. With multiple successes in preclinical research over the last decade, the Vevo® MD is FUJIFILM VisualSonics’ first foray into the clinical market.
 Source: FUJIFILM VisualSonics, Inc.
Source: FUJIFILM VisualSonics, Inc.
“This clearance represents another major accomplishment for FUJIFILM VisualSonics.” said Masayuki Higuchi, president & CEO, FUJIFILM SonoSite, Inc. “This powerful and innovative technology offers unprecedented image resolution capabilities that will have significant impact on the U.S. medical imaging community and the care providers deliver to patients.”
First commercialized in Europe, the Vevo MD is truly a unique ultrasound system, as it operates at much higher frequencies than any conventional ultrasound system currently available. It allows medical professionals to see what they have never seen before—unparalleled image resolution down to 30 micrometers. The Vevo MD system is compatible with FUJIFILM VisualSonics UHF series of transducers. This patented transducer technology is capable of operating in a range of frequencies up to 70 MHz, a tremendous increase in resolution compared to conventional ultrasound systems.
The Vevo MD was designed to play a role in a range of clinical application areas including neonatology, vascular, musculoskeletal, dermatology, and other small parts that are within the first 3 cm of the body.
“As the recognized leader in ultra high frequency imaging systems, FUJIFILM VisualSonics is proud to be the first to market with unparalleled technology.” said Renaud Maloberti, vice president & general manager of FUJIFILM VisualSonics. “With the Vevo MD, clinicians can observe the tiniest, most highly detailed anatomy that has never been seen before, which means greatly enhanced potential for diagnoses.”
“We are confident that Vevo MD is the kind of progressive tool U.S. health care providers will find to be of value for a wide array of applications as well as still unexplored areas.” said Andrew Needles, director of marketing at FUJIFILM VisualSonics. “Our hope is that this innovative new technology will lead to new medical discoveries, and ultimately, improved quality of patient care.”