Mar 10 2017
Researchers from Max Planck Florida Institute for Neuroscience designed new sensors to study the activity of signaling proteins in dendritic spines.
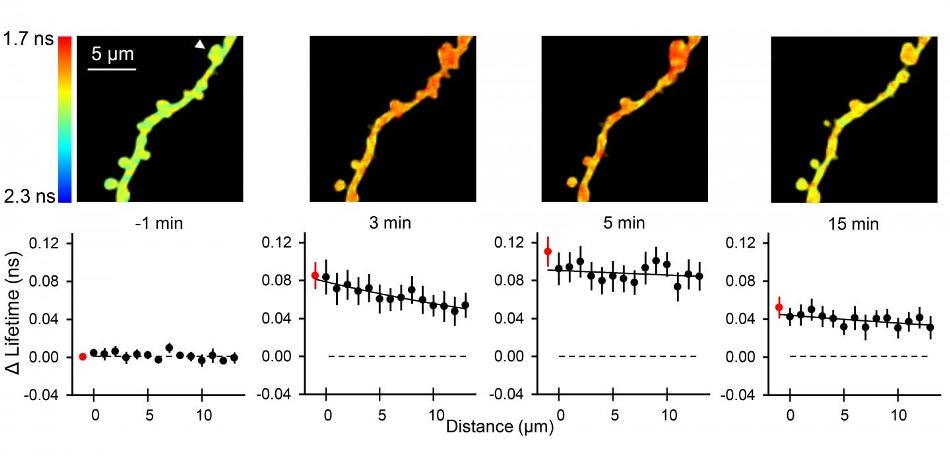 Top: Representative fluorescence lifetime images of ERK activation during glutamate uncaging. Stimulated spine was marked with the white arrowhead. Bottom: ERK activation during sLTP along the stimulated dendrite as a function of the distance from the base of the stimulated spine. (Credit: Max Planck Florida Institute for Neuroscience)
Top: Representative fluorescence lifetime images of ERK activation during glutamate uncaging. Stimulated spine was marked with the white arrowhead. Bottom: ERK activation during sLTP along the stimulated dendrite as a function of the distance from the base of the stimulated spine. (Credit: Max Planck Florida Institute for Neuroscience)
Learning and memory are crucial aspects of everyday life. When we learn, our neurons use chemical and molecular signals to change their shapes and strengthen connections between neurons, a process known as synaptic plasticity. In Ryohei Yasuda’s lab at Max Planck Florida Institute for Neuroscience (MPFI), scientists are working to understand how these molecules send messages throughout the neuron. To achieve this, his team is constantly working to develop high-resolution imaging techniques to visualize the activity and location of the molecules involved in the process. Ada Tang, Ph.D., a postdoctoral researcher in Yasuda’s lab, developed new molecular biosensors, which helped her visualize the activity of two signaling proteins crucial to synaptic plasticity, ERK and PKA. These proteins send messages to other proteins by adding a phosphate group to the target proteins. The team found that these proteins, which were already known to play a role in synaptic plasticity, learning, and memory, have surprising properties in their activity. The work was published in March 2017 in Neuron.
Dendrites are thin extensions that come out of a neuron’s cell body and receive messages from other neurons. They branch out to form a tree-like structure, each branch typically extending tens of micrometers. They are covered by spines: tiny protrusions that receive inputs from other neurons and initiate molecular signals inside the cell. When a spine is strongly stimulated, it grows and strengthens to encode memories. Scientists have previously used traditional pharmacological methods such as western blotting to determine the activity of ERK and PKA averaged over many cells, but they haven’t been able to visualize the molecules directly in dendritic spines because of their small size.
To design sensors sensitive enough to visualize these molecules, Tang created a new dye molecule, sREAChet, a modified dark but light-absorbing molecule. When she linked sREAChet with both green fluorescent protein (GFP) and a target peptide of the protein, she found that it could readout the activity of the protein with 2-3 times higher sensitivity compared to previous sensors. This made the sensitivity sufficient for imaging activity in single dendritic spines. “These sensors will be useful for researchers in a broad field of cell biology, since ERK and PKA are involved in a variety of phenomena in cells and their abnormal activity is related to many diseases including cancer and mental diseases,” explained Yasuda.
To demonstrate the usefulness of the new sensors, Yasuda’s team first stimulated individual dendritic spines, then used a special microscope called a 2-photon fluorescence lifetime microscope to visualize how ERK and PKA activity moves from a single spine. To their surprise, the team found the proteins’ activity did not stay within the individual spine, but spread much more than 10 micrometers, along the dendrite, influencing nearby spines. The spreading is estimated to be about several tens of micrometers and potentially extends throughout a branch of dendrites. The Yasuda Lab had previously shown that stimulating just a few spines could lead to ERK activation in the nucleus, but they didn’t know how this was achieved. This experiment showed that after these proteins are activated in a spine, the message spreads strongly over a long distance and potentially reaches the nucleus. “To find that PKA and ERK activation in spines is spreading for several tens of micrometers is certainly a surprising discovery for the field,” said Tang.
The team has visualized an important step in the process, but there is still a long way to go to understanding the biochemical underpinnings of learning and memory.
This work was supported by grants from National Institute of Health (R01MH111486, R01MH080047, and 1DP1NS096787) and the Max Planck Florida Institute for Neuroscience.