Jun 5 2018
The detection of biomolecules — such as nucleic acids, proteins, and lipids — as well as their interactions in heterogeneous biological samples is highly important for gaining insights into a large number of biological mechanisms in health and disease.
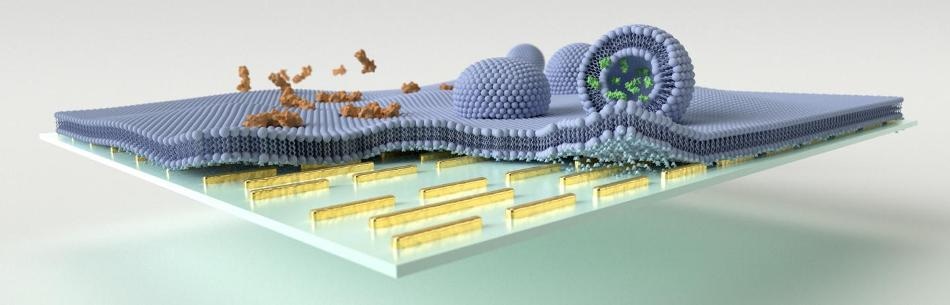 Multi-resonant mid-IR nanoantennas are leveraged to enhance the vibrational absorption signals associated with biomimetic lipid membrane formation, polypeptide/membrane interaction, and vesicular cargo release on the sensor surface. (Image credit: EPFL)
Multi-resonant mid-IR nanoantennas are leveraged to enhance the vibrational absorption signals associated with biomimetic lipid membrane formation, polypeptide/membrane interaction, and vesicular cargo release on the sensor surface. (Image credit: EPFL)
For example, transport in cells and molecular signaling are regulated by the association and insertion of proteins with the cell lipid membrane. Yet, with prevalent label-free methods, it is hard to differentiate protein insertion, membrane disruption, and chemical release processes, thereby pushing experimentalists to rely on various methods that often mandate distinctive experimental settings. Hence, it is vital to design innovative biosensors that have high selectivity and sensitivity and can leverage the chemical signature of distinctive biomolecular species to allow analysis of complex multi-analyte interactions.
In a study reported in the Nature Communications journal, scientists at Ecole Polytechnique Fédérale de Lausanne (Switzerland) and their collaborators from the United States have described a mid-infrared biosensor based on an innovative multi-resonant metasurface. For the first time, the biosensor has been able to non-destructively differentiate multiple analytes in heterogeneous biological samples, with high sensitivity and in real time. This is realized by the new sensor by accessing the unique chemical fingerprint information of peptides, lipids, proteins, or other biochemicals, enabling independent and simultaneous monitoring of their interaction dynamics.
Specifically, the study demonstrates the ability of the sensor to spectroscopically resolve the interaction between biomimetic lipid membranes and different peptides, and also the dynamics of vesicular cargo release. These are biologically crucial mass-preserving processes that cannot be accessed through standard label-free methods, irrespective of their sensitivity.
Astonishingly, the sensor has the ability to resolve the interaction of lipid membranes with a toxic pore-forming peptide, such as melittin, in supported membranes as well as surface-tethered vesicles loaded with neurotransmitter molecules. The study reports the real-time monitoring of melittin-induced membrane disruption and neurotransmitter cargo release from such synaptic vesicle mimics, without labeling and with monolayer sensitivity.
Such significant proof-of-concept experiments open the door for the application of these biosensors to analyze the molecular mechanisms which are the basis for crucial processes that have been associated with human diseases, such as membrane disruption and pore formation induced by protein aggregation in neurodegenerative diseases such as Parkinson’s and Alzheimer’s disease.
The new biosensor is a robust tool for the differentiation, identification, and simultaneous analysis of the interactions that take place among different biological species in complex samples, thereby overcoming the evident defects of prevalent label-free methods. Moreover, it can be applied to investigate a large number of multi-analyte biological systems, paving the way for fascinating applications in various fields such as fundamental biology, pharmaceutical drug development, and so on.