Sep 3 2018
Scientists at Tokyo Institute of Technology have developed a fluorescent protein sensor based on a protein from E. coli. This new sensor is capable of providing real-time information on dynamic variations in oxygen levels with extremely high sensitivity. As the oxygen level is considered to be a key determinant of cellular function, the concept behind this sensor may be able to revolutionize an individual’s potential to identify cellular changes of extreme importance, such as in tumors and following a heart attack or stroke.
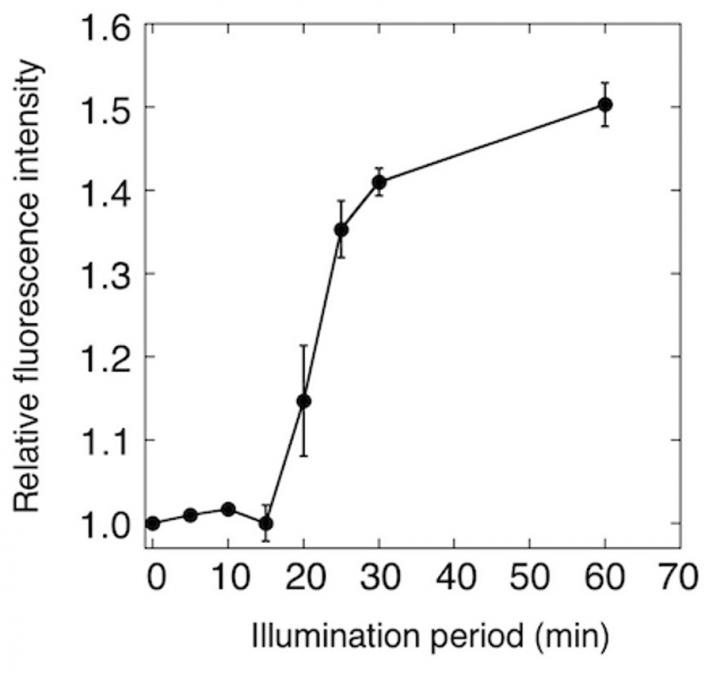 ANA sensor was added to the culture of cyanobacteria, and the fluorescence of ANA sensor was monitored. Fluorescence of ANA sensor started to increase after 15 minutes of illumination, indicating the ANA sensor detected oxygen produced by cyanobacteria. (Image credit: Toru Hisabori)
ANA sensor was added to the culture of cyanobacteria, and the fluorescence of ANA sensor was monitored. Fluorescence of ANA sensor started to increase after 15 minutes of illumination, indicating the ANA sensor detected oxygen produced by cyanobacteria. (Image credit: Toru Hisabori)
Oxygen plays a key role in the biochemical processes that bring about the possibility of life on earth. Being able to accurately and rapidly measure oxygen levels within living cells could be useful in many different areas of biology, including medicine, bioengineering, and physiology. For instance, oxygen levels in cancer cells can actually affect their response to anti-cancer therapies, whereas oxygen levels present in tissues following a heart attack or stroke can influence both treatment and recovery. In an article published recently in the journal Scientific Reports, Jiro Nomata and Toru Hisabori, researchers at Tokyo Institute of Technology, present a report on the development of a new kind of oxygen sensor capable of dramatically altering an individual’s potential to identify changes in the levels of cellular oxygen.
"Limitations in previously developed methods to measure oxygen levels make it difficult to analyze oxygen levels in living cells" notes Prof Hisabori, "so we aimed to overcome these limitations by developing a genetically encoded sensor that can provide real-time information on the dynamic changes of oxygen levels in living cells."
The researchers employed a protein known as the direct oxygen sensor protein (DosP) from the bacterium E. coli, which has the potential to either release or bind oxygen based on the oxygen levels present in the cell. Before examining the fluorescence intensity of the resulting product under varying oxygen levels, the part of the protein capable of binding oxygen was isolated and then linked to a fluorescent protein. The researchers discovered that the fluorescence of their novel protein, called ANA sensor (anaerobic/aerobic sensing fluorescence protein), decreased in the absence of oxygen and increased in the presence of oxygen, thus successfully tracking the dynamic variations in oxygen content.
Additional development allowed the researchers to further modify the protein to enable more precise quantification of oxygen levels. The ANA sensor was thus used for monitoring photosynthetic oxygen production by a photosynthetic microorganism (cyanobacteria). Notably, in a dramatic development over earlier oxygen detection methods, changes in oxygen levels are reflected by variations in ANA sensor fluorescence with extremely high sensitivity.
The most vital aspect of this study possibly refers to the potential of applying this method to the development of other protein sensor probes in order to detect several cellular changes at the molecular level. "Almost all current sensor protein probes are based on conformational changes," notes Dr. Nomata. "In contrast, the fluorescence quenching mechanism used in this study expands the possibilities for the development of novel protein sensor probes."