Bruin Biometrics, LLC (“BBI”) has been granted U.S. Food and Drug Administration (FDA) marketing authorisation for the SEM Scanner, becoming the world’s first FDA-authorised device to objectively alert clinicians to specific anatomical areas of a patient’s body at increased risk for developing pressure damage. Patient risk assessments are performed with the SEM Scanner before visible damage manifests at the skin surface – a world and clinical first.
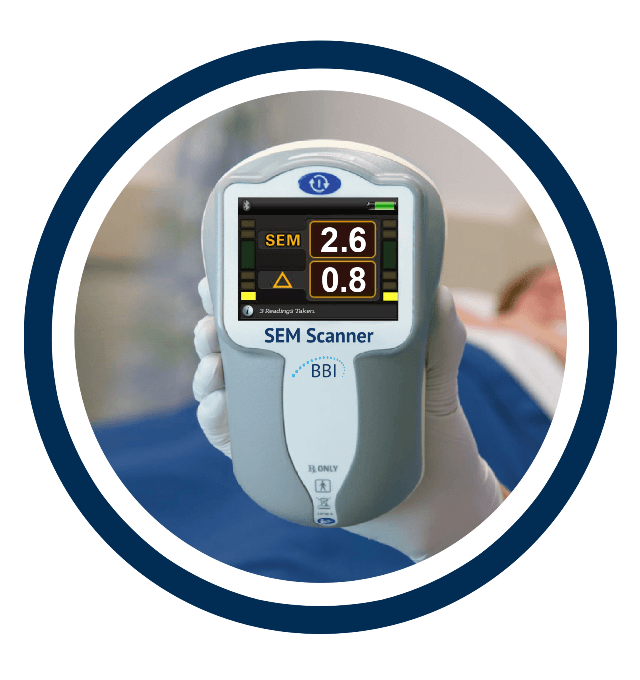
The SEM Scanner received European CE Mark approval in 2014 and Health Canada clearance in 2016 and is in full commercial use in Canada and the European Union including the UK, Belgium and Spain with additional markets expected to be opened in 2019.
FDA granted marketing authorisation for the SEM Scanner under its de novo review process for novel low- to moderate-risk devices that are not substantially equivalent to an already legally marketed device.
More than 2.5 million people annually develop bed sores in the United States, including nearly one out of ten patients in hospitals and almost one-third of patients in long-term acute care. The bed sores can lead to pain, disfigurement, infection and complications such as sepsis, cellulitis, and MRSA. 60,000 Americans die each year from complications from pressure ulcers – an equivalent mortality rate to the opioid crisis -- at an annual cost of up to $11.6 billion to the U.S. health care system. These injuries result from pressure and shear causing localised damage to the skin and underlying tissue, typically at areas of bony prominence, such as the heels and sacrum.
Internationally, Pressure Ulcers represent a significant clinical and financial burden with a prevalence of 8.3% to 23% dependent upon the country. In the UK for example, recent research from NHS Improvement shows that treating pressure damage costs the NHS more than £3.8m per day, and that 1700- 2000 patients per month develop pressure ulcers.
FDA authorisation was based, in part, on data from a clinical study assessing performance of the SEM Scanner compared to visual skin assessment by nurses in 182 patients at risk for pressure ulcers at 12 hospitals and skilled nursing facilities in the U.S. and United Kingdom.
BBI CEO Martin Burns welcomed the FDA decision: “Our singular objective is to reduce pressure injury incidence by helping clinicians make prevention real. Total prevention of avoidable pressure injuries is mathematically impossible under the current standard of care. Prevention success demands objective, early, anatomically specific data. For the first time, in the US, clinicians will have access to anatomically specific risk assessment data that can be gathered from increased risk patients in all care settings. We are optimistic of the impact these data will have on prevention strategies in the U.S.A.”
To date in the UK alone 20 different healthcare facilities have experience of using the SEM Scanner within a number of different healthcare settings including: Medical, Orthopaedic, Elderly care, Intensive Care, Vascular Care and End of Life Care. Individual NHS trusts and private providers have enjoyed and won numerous awards for innovation and patient safety, including the Health Service Journal’s Patient Safety Award for Innovation (2017) and the Journal of Wound Care’s Most Innovative Product (2018) for their use of the SEM Scanner.