Jan 11 2019
Electrochemical sensors and biosensors allow scientists to measure small amounts of chemicals or physico-chemical parameters in experimental sites. This is accomplished with the use of sensitive electrodes which can detect small variations in electrical signals. As a result of this sensitivity, they have varied applications in medicine and engineering. Newer versions of sensors provide better accuracy and sensitivity with the help of nanomaterials integrated in electrodes used in the sensor.
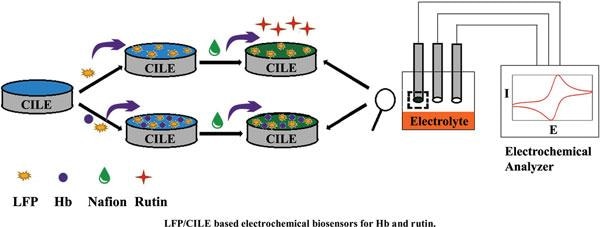 This is an application of nano-sized LiFePO4 modified electrode to electrochemical sensor and biosensor. (Image credit - Wei Sunf and Bentham Science Publishers)
This is an application of nano-sized LiFePO4 modified electrode to electrochemical sensor and biosensor. (Image credit - Wei Sunf and Bentham Science Publishers)
Scientists led by Wei Sun at the College of Chemistry and Chemical Engineering, Hainan Normal University have tested electrodes altered with Lithium Iron Phosphate (LFP) for biochemical analysis or rutin (a citrus flavonoid) and hemoglobin. According to the scientists, LFP is a favorable candidate to create new improved electrodes because of its advantages such an environmental compatibility, long cycle life, low cost, non-toxicity, high safety, and abundance in the environment.
Sun’s team employed scanning electron microscopy to differentiate nano-sized LFP particles. The LFP modified electrodes were then prepared by casting a solution of the particles over the surface of a Carbon Ionic Liquid Electrode (CILE) and incorporating drops of chitosan on the modified electrodes. Two individual electrodes were prepared for examining rutin and hemoglobin, respectively.
The researchers investigated the electrochemical activity of rutin and hemoglobin using these nano-LFP electrodes and realized detection limits of 8.0 nmol L-1 for rutin and, in the case of hemoglobin, 0.068 mmol L-1 for trichloroacetic acid reduction, and 0.07 μmol L-1 for hydrogen peroxide reduction.
This article by Dr. Wei Sunf et al. is published in Current Analytical Chemistry, Volume 14, Issue 5, 2018.