Feb 20 2019
A new wearable electromyography biofeedback device that connects to innovative smartphone games may provide people with incomplete paraplegia a more reasonably priced, self-controllable therapy to improve their recovery, according to new research presented recently at the Association of Academic Physiatrists Annual Meeting in Puerto Rico.
 (Image credit: Shirley Ryan AbilityLab)
(Image credit: Shirley Ryan AbilityLab)
Electromyography (recording electrical activity of muscles) biofeedback has been revealed to improve recovery of muscle control in patients with incomplete spinal cord injury. However, current biofeedback therapy devices are costly and can be worked only by skilled personnel in a laboratory setting. These factors prevent numerous people—nearly 50,000 in the US—from accessing the biofeedback therapy that could be advantageous for their recoveries.
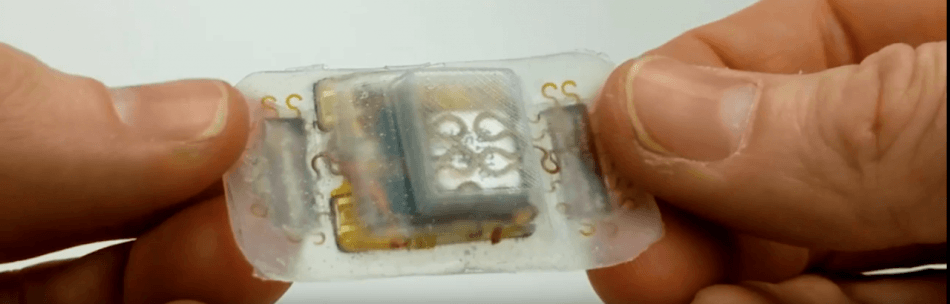
To help vanquish these hurdles, a team of scientists—led by R. James Cotton, M.D., Ph.D., at Shirley Ryan AbilityLab (previously the Rehabilitation Institute of Chicago) and John Rogers, Ph.D., at Northwestern University—designed an inexpensive, wearable-sensor biofeedback platform that enables people with incomplete paraplegia to self-dose this type of therapy.
Although various research systems can record muscle activity, only a few are portable and can be worn at ease over a few days. Unfortunately, none of these systems offer real-time access to the data via smartphones. The groundbreaking system offers this functionality, allowing biofeedback via games, which can be played away from the lab.
Shirley Ryan AbilityLab and Northwestern scientists deployed recent progress in flexible, stretchable electronics to build a wearable electromyography sensor. The device lets subjects to use movement and muscle activation to regulate innovative smartphone games—which were also put together by the scientists—making biofeedback easily and continuously available. Data from the new system, comprising muscle activity and game performance, is visibly synchronized to a safe cloud database, permitting monitoring by clinicians and scientists.
The device is stuck to the skin using conductive tape and employs integrated electrodes to record muscle activity. The low-profile sensor has Bluetooth connectivity, wireless charging, and a nine-axis inertial measurement unit. The battery functions for several days and may be charged wirelessly using low-cost commercial units.
Data collected by the research team from intact subjects has revealed stable measurements over time. Pilot data from subjects with spinal cord injuries confirm that the device has adequate sensitivity to detect muscle activation and to regulate the biofeedback games.
The developers believe that their new system for electromyography biofeedback beats many of the hurdles to extensive use of this therapeutic modality. Analysis of the device is continuing to establish if self-dosed biofeedback can improve recovery of electromyography activity and other functional outcome measures. Right now, the device is still to get FDA approval.
The Craig H. Neilsen Foundation generously supported this research.
New Wearable Sensor for People with Incomplete Paraplegia