Mar 5 2019
Even meager quantities of water can now be detected by a versatile, innovative plastic-composite sensor. The 3D-printable material, designed by a Spanish-Israeli group of researchers, is flexible, inexpensive, and non-toxic and changes its color from purple to blue under moist conditions.
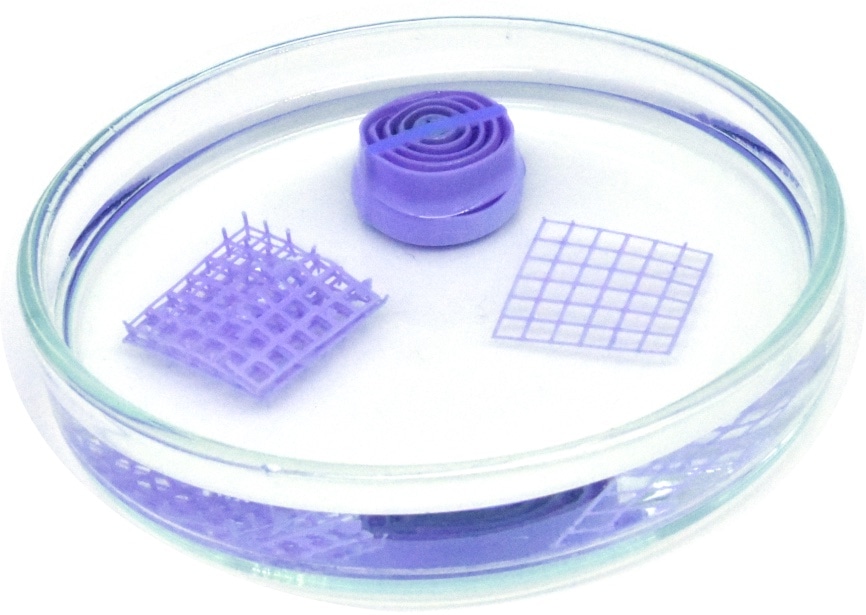 When dried, for example in a water-free solvent, the sensor material turns purple. (Image credit: UAM, Veronica Garcia Vegas)
When dried, for example in a water-free solvent, the sensor material turns purple. (Image credit: UAM, Veronica Garcia Vegas)
Headed by Pilar Amo-Ochoa from the Autonomous University of Madrid (UAM), the scientists used DESY’s X-ray light source PETRA III to gain insights into the structural variations within the material initiated by water and resulted in the observed color change. The advancement paves the way for the era of a class of innovative 3D-printable functional materials, as described by the researchers in the Advanced Functional Materials journal (early online view).
In various fields, such as health to food quality control, technical applications, and environmental monitoring, there is an ever-increasing requirement for responsive sensors that display simple and fast changes in the presence of particular molecules. One of the most common chemicals to be monitored is water.
Understanding how much water is present in a certain environment or material is important. For example, if there is too much water in oils they may not lubricate machines well, whilst with too much water in fuel, it may not burn properly.
Michael Wharmby, Scientist, DESY
Wharmby is the co-author of the paper and also the head of beamline P02.1, where the sensor material was investigated using X-rays.
The functional part of the new sensor material developed by the researchers is a so-called copper-based coordination polymer, a compound in which a water molecule is bound to a central copper atom.
On heating the compound to 60 degrees Celsius, it changes colour from blue to purple. This change can be reversed by leaving it in air, putting it in water, or putting it in a solvent with trace amounts of water in it.
Pilar Amo-Ochoa, Autonomous University of Madrid
The researchers used high-energy X-rays from DESY’s research light source PETRA III at the experimental station P02.1 and could observe that when the sample was heated to a temperature of 60 °C, the water molecule attached to the copper atoms was removed. This results in a reversible structural reorganization of the material, which is the reason for the change in color.
“Having understood this, we were able to model the physics of this change,” explained co-author José Ignacio Martínez from the Institute for Materials Science in Madrid (ICMM-CSIC). Then, the researchers could mix the copper compound with a 3D printing ink and use it to print sensors of various different shapes. They tested the printed sensors in air and using solvents with different amounts of water. The test results revealed that the printed objects are much more sensitive to water compared to the compound due to their porous nature. In the case of solvents, the sensors could already detect 0.3%–4% of water within just two minutes. In the case of air, they were able to detect a relative humidity of 7%.
Upon being dried by heating or in a water-free solvent, the material becomes purple again. A thorough examination revealed that the material is stable even after several heating cycles, and the copper compounds are distributed evenly throughout the printed sensors. Moreover, the material is stable in air for at least one year as well as at biologically relevant pH ranges of 5–7. “Furthermore, the highly versatile nature of modern 3D printing means that these devices could be used in a huge range of different places,” reiterated co-author Shlomo Magdassi from The Hebrew University of Jerusalem. He further stated that the concept could be applied to create even other functional materials.
This work shows the first 3D printed composite objects created from a non-porous coordination polymer. It opens the door to the use of this large family of compounds that are easy to synthesize and exhibit interesting magnetic, conductive and optical properties, in the field of functional 3D printing.
Félix Zamora, Study Co-Author, Autonomous University of Madrid
The study was supported by the Autonomous University of Madrid, the Hebrew University of Jerusalem, the Nanyang Technological University in Singapore, the Institute for Materials Science in Madrid, and DESY.