A paper-based sensor for identifying even small volumes of hydrogen peroxide has been developed by a research team from the Indian Institute of Science (IISc). From healthcare and household products, this chemical is used in a wide range of applications, from hand sanitizer as a disinfectant to rocket fuel as a propellant, and it is also found in biological cells.
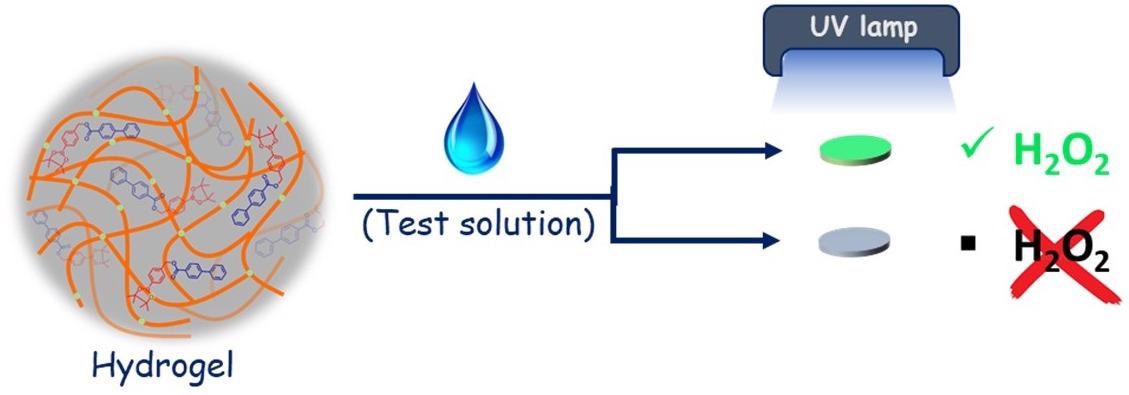 Schematic depicting the process to detect hydrogen peroxide Image Credits: Arnab Dutta.
Schematic depicting the process to detect hydrogen peroxide Image Credits: Arnab Dutta.
The procedure used by the researchers includes formulating a gel from a solution that has an exclusively designed molecule, which is treated with a hydrogen peroxide-containing liquid, and air-drying it on a thin paper disc that is approximately 0.45 cm in diameter.
When positioned under a UV lamp, the paper disc emits green light if hydrogen peroxide is present. The light intensity was discovered to be directly proportional to the hydrogen peroxide concentration.
You can actually visualize this green emission (photoluminescence) with the naked eye. You don’t need any sophisticated instruments. All you need is a simple UV light source.
Arnab Dutta, Study First Author and PhD Student, Department of Organic Chemistry, Indian Institute of Science
The study published was published in the journal ACS Sensors.
The paper disc can be a potential tool in low-resource environments, even for assessing biological fluids like blood, as it is economical, decomposable and easy to use. In other fields, efficiently detecting hydrogen peroxide is also essential. For example, peroxide-based explosives can be traced with hydrogen peroxide that is — at times — employed as a starting material.
When the team used their technique to randomly assess five different hand sanitizer brands, it was discovered that only three out of the five had the hydrogen peroxide level authorized by the World Health Organization, which is 0.125%. The fourth sanitizer brand had much lower than 0.125%, and the last had nearly zero hydrogen peroxide.
Hydrogen peroxide can be detected on a larger scale using titration and other experiments, but those are cumbersome and require training. This method is easy because of its simplicity.
Uday Maitra, Study Senior Author and Professor, Department of Organic Chemistry, Indian Institute of Science
Maitra’s lab has been at work to develop numerous “sensitizer” molecules that turn on the lanthanides — photoluminescence — of elements in the presence of certain chemicals or compounds. Previously, the team developed paper-based sensors for identifying certain antioxidants present in green tea — while eventually assessing its quality — and detecting sensors for different enzymes.
The sensitizer molecule designed by the researchers in this study allows terbium metal to emit green light when placed under a UV lamp. The green light disappears once the sensitizer and a masking agent are combined.
As soon as the hydrogen peroxide is added to this combination, it unmasks the sensitizer molecule and makes it glow green once again.
The way we designed the mask, that is where the thinking process comes in. The molecule we have designed is very specifically unmasked by hydrogen peroxide.
Uday Maitra, Study Senior Author and Professor, Department of Organic Chemistry, Indian Institute of Science
The researchers are now working to cut down the reaction time. If the hydrogen peroxide concentration is lower, the reaction time takes a bit longer.
Maitra states that the team is also working on developing a compact portable device, with which the detection could be further automated. “We are in touch with a start-up company in Chennai. We have a few prototypes made with UV LEDs and a camera, to generate the emission, take a photograph, and use an image processing app to quantify the amount of hydrogen peroxide,” he concludes.
Journal Reference:
Dutta, A. & Maitra, U. (2022) Naked-eye detection of hydrogen peroxide on photoluminescent paper discs. ACS Sensors. doi/10.1021/acssensors.1c02322.