Reviewed by Alex SmithJul 25 2022
Recently, scientists from the Chinese Academy of Sciences’ Suzhou Institute of Biomedical Engineering and Technology (SIBET) suggested a method for hand-in-hand structured DNA assembly.
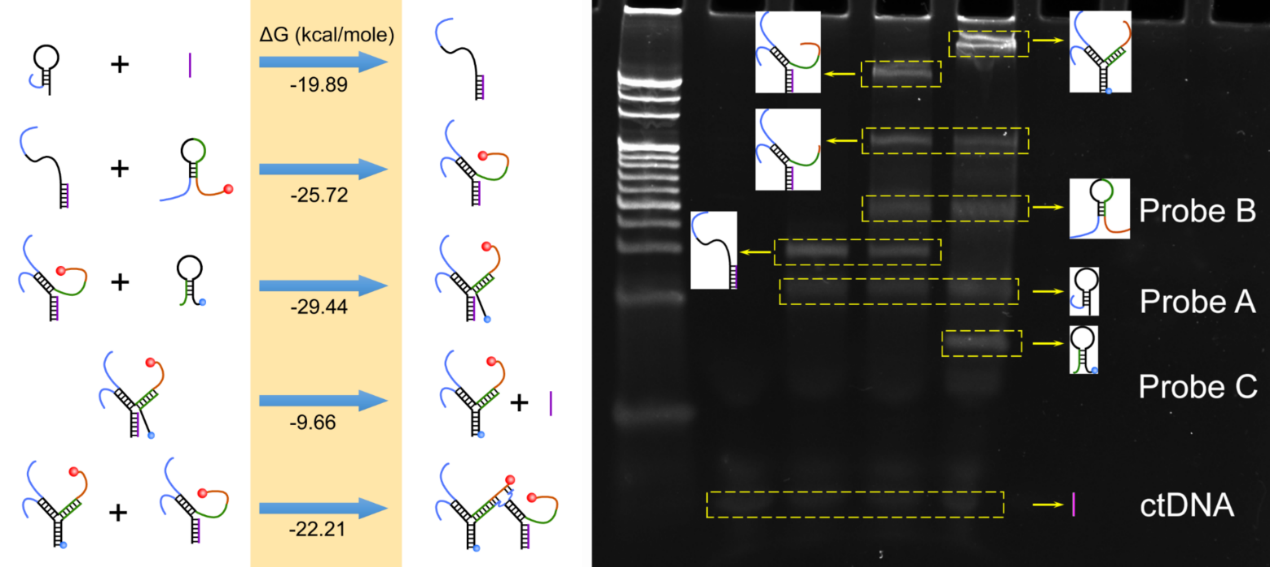 Standard free energy (ΔG) of reaction processes and PAGE analysis. Image Credit: Suzhou Institute of Biomedical Engineering and Technology (SIBET)
Standard free energy (ΔG) of reaction processes and PAGE analysis. Image Credit: Suzhou Institute of Biomedical Engineering and Technology (SIBET)
They have also created an electrochemical/fluorescent dual-mode biosensor for circulating tumor DNA based on methylene blue- and red-emissive carbon nanodots.
According to the researchers, the novel sensor combines the qualities of electrochemical and fluorescent sensors, whose signal sources and construction methods are typically very distinct.
A qualitative or quantitative approach based on the relationship between concentration or other physical characteristics and the electrical signal change generated by the target is known as an electrochemical sensor.
A fluorescence sensor is a device that detects things qualitatively or quantitatively by sending a particular target and recognition combination to a fluorescence element, which changes the fluorescence’s intensity or emission wavelength.
Integrating the two technologies for synchronous detection in one unique system can not only effectively improve the detection accuracy, but also reduce the influence of background signals, instrument fluctuations, and other factors on the obtained signals.
Peng Miao, Study Lead Researcher and Scientist, Suzhou Institute of Biomedical Engineering and Technology
In this sensor, Probe A is specifically tagged at its 5’ terminal, which immobilizes it at the electrode surface. Complete double strands are created between the target and its binding domain in Probe A when the target is present.
Meanwhile, the stem region is opened, and a single-stranded area is released, which opens Probe B’s second hairpin. Previously, it has been coupled to a 3’ terminal NH2 of a red-emitting carbon nanodot. When Probe B’s single-stranded area is liberated, Probe C’s hairpin structure is then unlocked.
Furthermore, Probe C can move the target sequence to create a full three-way junction structure.
Thus, the target is reused, helping to create a number of three-way intersections. Since the 3’ terminal of Probe C contains a large number of methylene blue molecules that are close to the electrode contact, a considerable electrochemical response might be recorded to identify the target.
The 3’ terminal of Probe B on the adjacent three-way junction serves as the substrate, and the released single-stranded swing arm created between the 3’ terminal of Probe A and the 5’ terminal of Probe B on the three-way junction serves as the DNAzyme. Thus, a coexisting structured DNA monolayer is created.
Mg2+ allows for the cleavage of the substrate sequence and the release of the conjugated carbon nanodots into the solution.
Miao stated, “By measuring the increased fluorescence emission, original target level can also be evaluated by fluorescence technique.”
The feasibility of the process was established by theoretical calculations and gel electrophoresis imaging. The synthetic carbon nanodots can retain excellent fluorescence stability and strong anti-interference in a physiological setting.
The linear calibration curves of electrochemical/fluorescence intensities and target concentration were produced by Miao and his team through a series of condition optimization and quantitative tests. These curves can reach a broad linear range of six orders of magnitude.
Fluorescence imaging also makes it simple to discern the target’s composition. The dual-mode sensor created in this study is innovative, has great sensitivity, and is highly scalable, making it an effective tool for clinical diagnostics and nucleic acid analysis.
The sensor is anticipated to be utilized extensively in several domains, including clinical trials, environmental detection, and fundamental research.
Journal Reference:
Liu, G., et al. (2022) Hand-in-hand structured DNA monolayer for dual-mode analysis of circulating tumor DNA. Chemical Engineering Journal. doi:10.1016/j.cej.2022.138069.