A newly performed study detecting dopamine has been headed by Dr. Qunyan Zhu (Changchun Institute of Applied Chemistry, Chinese Academy of Sciences).
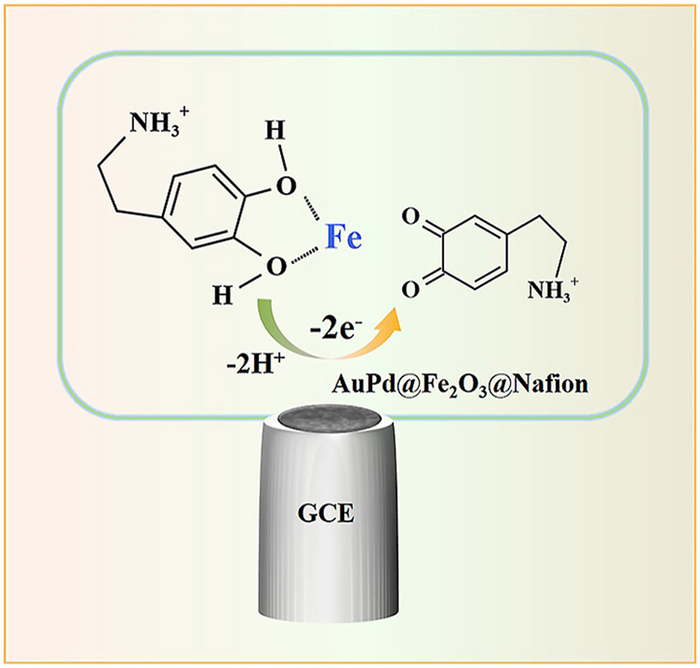
The AuPd@Fe2O3 NPs-based EC sensor (AuPd@Fe2O3 NPs/GCE) was prepared conveniently by dropping the mixture of AuPd@Fe2O3 NPs and Nafion on glassy carbon electrode (GCE), and firstly utilized for the detection of DA. Art by Zhu’s group. Image Credit: Changchun Institute of Applied Chemistry, Chinese Academy of Sciences
Dopamine (DA) is a significant neurotransmitter that impacts the central nervous system by controlling numerous functions such as learning, memory, cognition, nervous system endocrine, and motor control.
Abnormal levels of DA present in biological fluids are largely linked to affective disorders, learning disabilities, Huntington’s disease, Parkinson’s disease, etc. Hence, it is significant to precisely measure the number of active ingredients in DA-related drugs and also the level and fluctuation of DA present in human body fluids.
In the past few years, several methods have been developed for the detection of DA, such as electrochemical (EC) sensors, optical assays, capillary electrophoresis, mass spectrometry, etc.
Among such techniques, EC sensors have various benefits, like quick response speed, simple miniaturization, and easy operation steps. But in normal human body fluids, the physiological levels of DA are much lower (c.a., 100 to 1000 times) compared to those of ascorbic acid (AA), the significant interferer at the time of the EC measurement of DA.
The phenomenon means that the DA EC sensors should exhibit excellent selectivity and sensitivity. As a result of the slow electron transfer, the unaltered electrode is normally incapable of selectively and effectively detecting DA. For these drawbacks to be overcome, the electrode surface requires alteration with an ideal material with special EC properties.
So far, several materials such as metal-organic frameworks, metal nanoparticles (NPs), conducting polymers, metal complexes, and carbon-based materials have been utilized to alter electrode surfaces for the improvement of the EC detection performance of DA.
For instance, Song et al. made a DA-imprinted chitosan film electrode, which offered ample active sites for the detection of DA. Simultaneously, the electrode pores can be selective to DA. The interference of AA could be removed easily by applying nanomaterial-modified electrodes.
With the help of the Pt3Ni nanoalloy-modified GCE, the present signal response of the DA could be differentiated easily from those of AA and also other interfering substances.
Being one of the catechols, the phenolic hydroxyl group of DA has the potential to produce a quinone or semiquinone structure via the oxidation pathway of losing one electron or two electrons, respectively.
Depending on the molecular structure, the reaction principle of the EC oxidation reaction process of DA could be streamlined as the oxidation process of two adjacent hydroxyl groups (-OH) on the benzene ring to ketone groups.
As a result of their abundant active sites and excellent lattice conductivity, transition metals exhibit good electrocatalytic activity for the oxidation of hydroxyl functional groups. Iron oxide NPs and also their hybrids have gained so much attention in the fabrication of non-enzymatic EC sensors due to their stable physicochemical properties, tunable band gaps, and outstanding electrocatalytic performance.
But, there are some examples of the application of iron oxide NPs and their hybrids-modified electrodes used for the detection of DA in complex samples.
In this study performed, AuPd@Fe2O3 NPs with outstanding catalytic performance were made by a facile one-pot technique at room temperature. The AuPd@Fe2O3 NPs-based EC sensor (AuPd@Fe2O3 NPs/GCE) was made in a comfortable manner by dropping the mixture of AuPd@Fe2O3 NPs and Nafion on glassy carbon electrode (GCE) and initially used for the detection of DA.
In comparison with bare GCE, the as-prepared AuPd@Fe2O3 NPs/GCE displays an anti-interference for the detection of DA by chronoamperometry measurement, which could be utilized to precisely identify the levels of DA in actual biological samples and pharmaceuticals.
Journal Reference
Dai, M., et al. (2023) Sensitive and selective electrochemical sensor for the detection of dopamine by using AuPd@Fe2O3 nanoparticles as catalyst. Advanced Sensor and Energy Materials. doi.org/10.1016/j.asems.2023.100048.