How can brain cells, or neurons, distinguish between their own processes and those of other neurons while they are putting out processes to connect with other neurons? Clustered protocadherin (Pcdh), a molecule, is a crucial piece of this jigsaw.
Detection of self-recognition by IPAD. Image Credit: Takashi Kanadome
Researchers from SANKEN (The Institute of Scientific and Industrial Research) and Osaka University’s Graduate School of Frontier Biosciences reported the development of a sensor to look at Pcdh interactions in live neurons in a recent publication in iScience, which helped solve this mystery.
Millions of neurons in the brain form trillions of connections with one another. To do this, each neuron releases microscopic processes that spread outward and locate the processes of neighboring cells to connect with.
Cells could unintentionally link with themselves rather than with other cells since they have so many activities going on inside of them. Pcdh, which is expressed in various combinations on the surface of each neuron, is one strategy to prevent this.
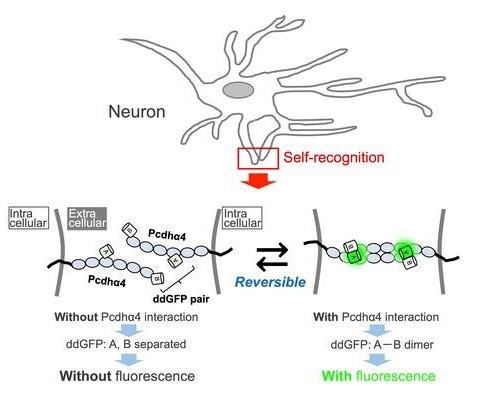
Cell adhesion is one function of Pcdh; if two neuronal processes contain precisely the same mix of Pcdh molecules, the molecules adhere to one another. On the other hand, if there is even a small difference between the combinations, they are seen as “other” rather than “self,” and do not bond.
Although there are established methods for identifying molecular interactions across cell surfaces, these methods only provide information about the times when molecules bind and dissociate. Osaka University researchers intended to address this issue.
We developed a fluorescent-based sensor that we named IPAD, or Indicators for Protocadherin Alpha 4 interactions upon Dimerization. This sensor allows us to see not only interactions between processes, but also the dissociation of these interactions for the first time.
Takashi Kanadome, Study Lead Author and Researcher, Osaka University
This novel method does have certain drawbacks. For instance, it is unable to distinguish connections between processes from the same cell and those from two separate cells with the same combinations of Pcdh on the surface, and its fluorescence is significantly duller than that seen using previous techniques.
Despite its current drawbacks, we think that our new sensor will be useful for a number of different research applications. The development of IPAD is an important step toward a better understanding of the neuronal recognition of self/other.
Tomoki Matsuda, Study Senior Author and Assistant Professor, Osaka University
The study’s sensors offer a wide range of possible uses. The method might be used, for example, to create a variety of fluorescent sensors to observe neuronal self-connectivity, which is thought to play a role in neurological disorders, including autism and epilepsy.
The ability to treat these diseases more effectively could be influenced by a deeper understanding of neuronal self-connectivity.
Journal Reference
Kanadome, T., et al. (2023) Visualization of trans-interactions of a protocadherin-α between processes originating from single neurons. iScience. doi:10.1016/j.isci.2023.107238.