In a recent article published in the journal Nature Communications, researchers from the US and Singapore introduced a novel nanosensor platform that can monitor the early stress signaling molecules in living plants.
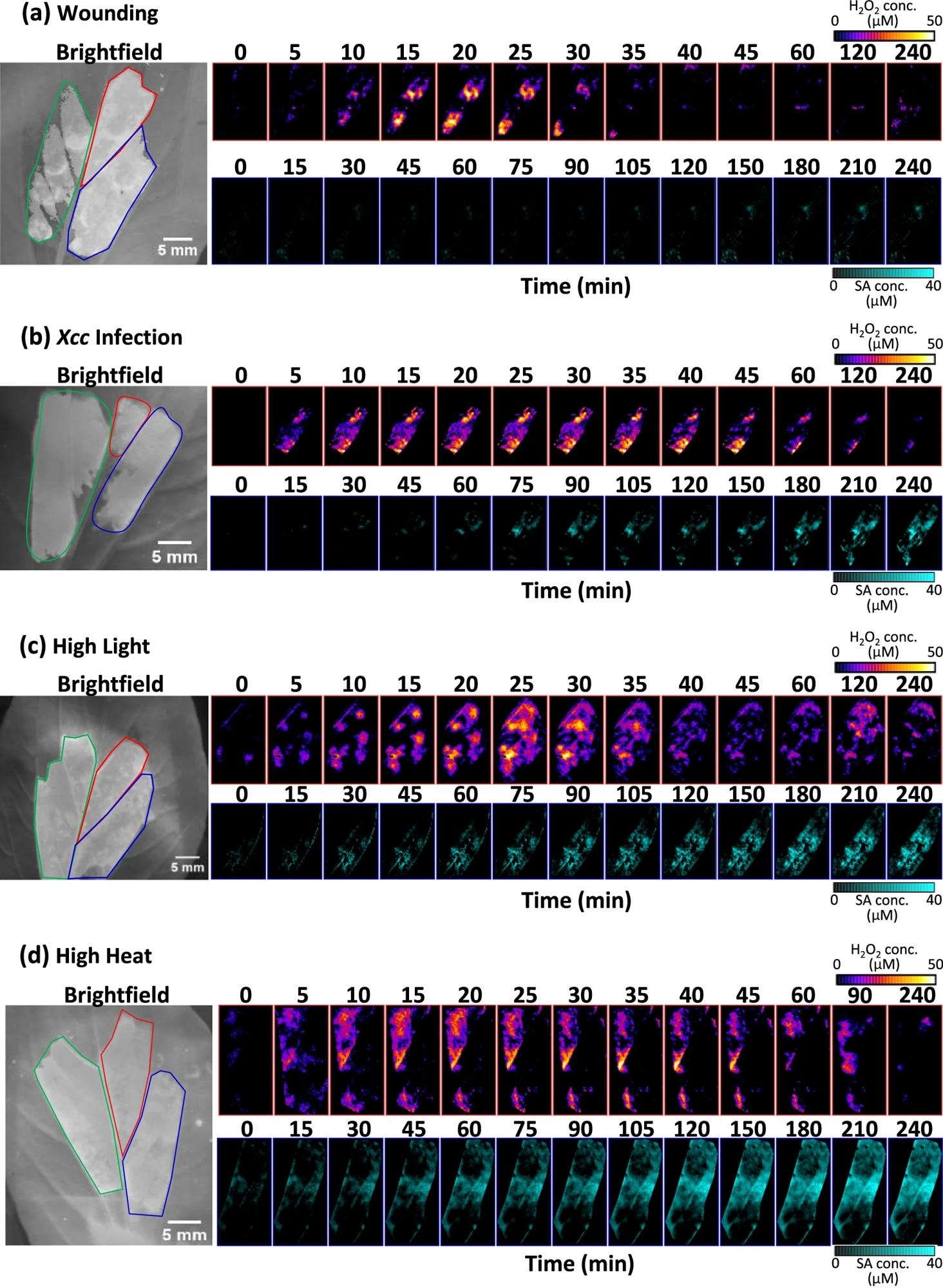 Pak choi plants were subjected to (a) mechanical wounding, (b) Xcc infection, (c) high light, and (d) high heat treatment, respectively. Snapshots of H2O2 concentration maps are shown at 5-min intervals for the first 45 min post-stress capturing the first ROS wave, followed by 1 h, 1.5, or 2 h, and 4 h time-points capturing the secondary ROS wave. Snapshots of SA concentration maps are shown at 15-min intervals for the first 2 h post-stress, followed by 30-min intervals for the next 2 h. Image Credit: https://www.nature.com/articles/s41467-024-47082-1
Pak choi plants were subjected to (a) mechanical wounding, (b) Xcc infection, (c) high light, and (d) high heat treatment, respectively. Snapshots of H2O2 concentration maps are shown at 5-min intervals for the first 45 min post-stress capturing the first ROS wave, followed by 1 h, 1.5, or 2 h, and 4 h time-points capturing the secondary ROS wave. Snapshots of SA concentration maps are shown at 15-min intervals for the first 2 h post-stress, followed by 30-min intervals for the next 2 h. Image Credit: https://www.nature.com/articles/s41467-024-47082-1
They utilized carbon nanotubes wrapped with polymers to detect key molecules involved in plant stress responses, including hydrogen peroxide (H2O2) and salicylic acid (SA). The research demonstrated the potential of nanosensor multiplexing as a powerful tool for elucidating plant stress signaling mechanisms and developing climate-resilient crops.
Background
Plants are often subjected to various environmental stresses, such as drought, heat, pathogens, and physical damage, which impact their growth and productivity. In response to these challenges, plants initiate complex signaling pathways involving a multitude of molecules, including reactive oxygen species (ROS) and plant hormones. Among these molecules, H2O2 stands out as a crucial redox molecule, serving as a rapid and systemic signal for perceiving and responding to stress.
Among plant hormones, SA plays a multifaceted role in regulating numerous aspects of plant growth, development, and immunity. It is particularly important for mediating the plant's response to pathogen infections and inducing systemic acquired resistance (SAR), which provides broad-spectrum immunity against future attacks. However, precisely understanding the timing and sequence of events in the H2O2 and SA cascade is still a challenge. This difficulty stems from the current limitations in non-destructive, real-time detection technologies needed to monitor these molecules within living plants.
About the Research
In this study, the authors proposed a nanosensor platform utilizing single-walled carbon nanotubes (SWNTs) for seamlessly monitoring stress responses in plants. SWNTs were selected for their remarkable photostability and fluorescence in the near-infrared (nIR) range, effectively minimizing interference from chlorophyll auto-fluorescence. By coating SWNTs with specific polymers, the researchers developed sensors capable of selectively binding to H2O2 and SA, thereby modulating their nIR fluorescence.
The H2O2 sensor utilized SWNT coated with a single-stranded deoxyribonucleic acid (DNA) oligomer featuring 15 repeats of the guanine-thymine nucleotide sequence ((GT)15), providing it with a specific binding affinity to H2O2. In contrast, the SA sensor was developed through the screening of various cationic fluorene-based co-polymers as SWNT coatings to identify the one exhibiting the most pronounced fluorescence quenching response upon binding to SA.
Furthermore, the efficacy of these sensors was confirmed through validation in transgenic Arabidopsis thaliana plants, which can accumulate diverse levels of SA following estradiol induction. Notably, these sensors were found to localize within crucial cellular compartments such as the cytoplasm, chloroplast, and apoplast, where SA biosynthesis and signaling processes occur.
Furthermore, the nanosensors were employed to monitor the spatiotemporal dynamics of H2O2 and SA production in pak choi plants under different stress conditions, including light exposure, heat, pathogen attack, and mechanical wounding.
Research Findings
The outcomes showed that each stress treatment triggered different waves of H2O2 and SA in pak choi leaves. The H2O2 wave served as the initial and immediate signal, emerging within minutes of stress application and peaking within the first hour. Its characteristics, including intensity, duration, and speed, varied depending on the stress type. In contrast, the SA wave exhibited a delayed onset and a slower accumulation rate compared to H2O2. The timing of SA production also differed based on the stress type, suggesting diverse plant responses preceding the decline of the H2O2 wave.
Additionally, the authors observed interactions between the H2O2 and SA pathways, including positive or negative feedback loops, under different stress conditions. For instance, high heat stress induced a positive feedback loop between H2O2 and SA, leading to sustained elevation of both molecules. However, high light stress triggered a negative feedback loop, wherein SA inhibited H2O2 production and vice versa. Based on these observations, the study proposed a biochemical kinetic model suggesting that the early H2O2 signal carries stress-specific information, influencing subsequent SA production.
Applications
The newly introduced nanosensor offers real-time, non-invasive, and precise insights into the dynamics and interplay of stress signaling molecules when plants encounter various environmental stimuli. This technology holds promise in uncovering the complex molecular mechanisms and regulatory networks underlying plant stress responses and resilience.
Moreover, the platform's ability to diagnose plant stress accurately before symptoms appear enables timely interventions, mitigating yield losses. By expanding its capabilities to detect additional plant hormones and signaling molecules like jasmonic acid, abscisic acid, and nitric oxide, the platform can provide a detailed understanding of stress responses across different plant species and crops. This advancement contributes to the development of climate-resilient and stress-tolerant plants, crucial for ensuring food security and sustainable agriculture in the face of changing environmental conditions.
Conclusion
In summary, the novel sensor proved to be efficient and robust for effectively decoding early stress signaling molecules H2O2 and SA in living plants. By multiplexing the nanosensors, the authors revealed the distinct temporal patterns and interactions of H2O2 and SA under different biotic and abiotic stresses.
The researchers acknowledged some limitations and challenges, including concerns regarding the sensor's stability, sensitivity, and specificity. They also identified difficulties in scaling up sensor production and infiltration methods. Moving forward, they proposed directions for future work, which includes the development of additional nanosensors for detecting other plant analytes, enhancing methods for sensor delivery and localization, and integrating sensor data with mathematical models and machine learning algorithms.
Journal Reference
Ang, M.CY., Saju, J.M., Porter, T.K. et al. Decoding early stress signaling waves in living plants using nanosensor multiplexing. Nat Commun 15, 2943 (2024). https://doi.org/10.1038/s41467-024-47082-1, https://www.nature.com/articles/s41467-024-47082-1.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.