BioVigilant Systems has introduced its advanced series of high-speed biologic detection systems for monitoring air environment in pharmaceutical production areas.
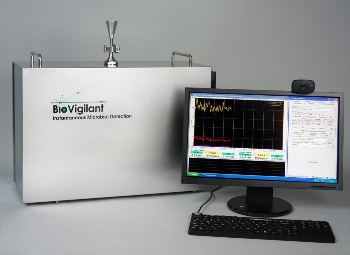 IMD-A 300 system
IMD-A 300 system
The IMD-A 300 and the IMD-A 350 systems can quickly identify the presence of bacteria instantly during the drug production process when compared to traditional growth methods, which nearly take a week to detect bacteria. Therefore, the IMD-A systems can speed up the quality assurance procedures for releasing drug batches.
The IMD-A 300/350 systems use laser light to distinguish between inert and biologic particles on a single particle analysis basis. The single particle analysis ability allows the system to detect bacteria with higher sensitivity compared to traditional equipment that displays restrained operational ranges. Furthermore, the IMD-A systems are utilized to examine the environment continuously, while traditional methods are used to monitor the environment periodically.
The IMD-A 300 and the IMD-A 350 sample 1.15 liters and 28.3 liters of air per minute, respectively. Biologic particles and inert are gathered into any one of six bins ranging from 0.5 µ to more than 10 µ as presented in the systems’ software, PharmaMaster. The 21CFR Part-11complaint software in the IMD-A systems provides various levels of functionality according to the requirement of users and allows them to create a collection of profiles.
Aric Meares, CEO of BioVigilant, said that the IMD-A systems present a perfect tool to advance the Process Analytical Technology and Quality by Design principles in pharmaceutical production.