Masimo has declared that the voluntary product recall imposed on the Pronto-7 portable device’ noninvasive finger sensors have been lifted. This device is yet to receive FDA 510(k) clearance in the US.
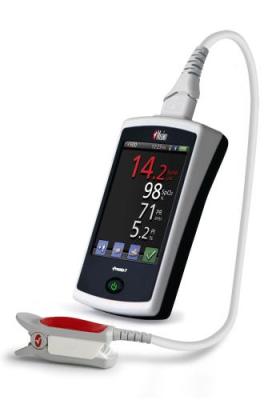 The palm-sized Pronto-7, with dimensions of just 13 cm x 7.2 cm x 2.5 cm (5.1" x 2.8" x 1") and weight of 296 grams (10.5 ounces), offers a breakthrough solution for measuring hemoglobin, SpO2, pulse rate, and perfusion index in less than one minute-without the needles, time-consuming laboratory analysis, risk of blood contamination, hazardous medical waste, and patient discomfort associated with traditional blood tests. Credit: Masimo
The palm-sized Pronto-7, with dimensions of just 13 cm x 7.2 cm x 2.5 cm (5.1" x 2.8" x 1") and weight of 296 grams (10.5 ounces), offers a breakthrough solution for measuring hemoglobin, SpO2, pulse rate, and perfusion index in less than one minute-without the needles, time-consuming laboratory analysis, risk of blood contamination, hazardous medical waste, and patient discomfort associated with traditional blood tests. Credit: Masimo
The product now includes new enhancements and features such as the Max Sensitivity Mode, which would permit measurements for a wider range of patients. Three noninvasive sensor sizes have been added which allow the usage of the instrument over a range of finger diameters. To identify the size of the sensors all three of them are color coded. The colors designated include large-white, medium-red and small-yellow.
Joe Kiani, the CEO and Founder of Masimo has revealed they had worked during the recall time until they had totally redesigned the sensor used in Pronto-7 so that it could be used for a range of ambient temperatures. This would offer healthcare providers the opportunity to transform the way care is provided to patients. The clinicians would be able to evaluate more number of patients and also make more informed decisions based on the noninvasive and speedy hemoglobin measurements provided by the Pronto-7.
More than 14,443 measurements were taken with the help of the Pronto-7 for carrying out the testing and verification processes before actual product launch. These measurements were taken from 14 sites and from 1443 patients, and then they were verified with hemoglobin measurements from sample of venous blood which was analyzed on a Coulter Counter or Hematology analyzer. The results of this evaluation proved to be far superior to the testing carried out before the Pronto-7 was recalled, and led to a lot of positive feedback from clinicians. The Pronto-7 would be available in Asia (except in Japan), South America, Africa, Middle East and in Europe.