A new study conducted by researchers at the Seattle-based University of Washington School of Medicine, published in the recent issue of Cell Press’ journal Immunity, titled “Autoimmunity Initiates in Nonhematopoietic Cells and Progresses via Lymphocytes in an Interferon-Dependent Autoimmune Disease” has provided vital insights into the development of autoimmune diseases.
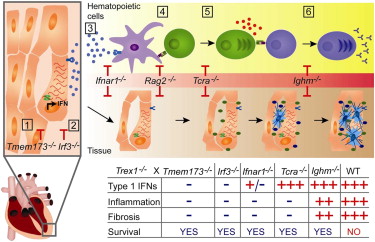 Graphical Abstract of the AutoImmunity Study
Graphical Abstract of the AutoImmunity Study
The study explained using a mouse model of the autoimmune disease in humans; abnormalities in intracellular sensors capable of detecting invading viruses and consequently leading to autoimmune pathology have been revealed.
In human beings, several intracellular sensors are present that are capable of detecting infections caused by viruses by binding to the DNA and RNA of the invading virus. The duplication of the viruses is prevented by the presence of receptors that activate IFN cytokines and enable the body to provide immunity.
Dr. Daniel B Stetson, an assistant professor in the university’s department of immunology and the senior author of the study, stated that sensors triggered an interferon response that is vital in protecting from infection. However, the response has to be regulated carefully to ensure that the cells RNA and DNA are prevented from unsuitable activation.
In previous studies, the researchers identified Trex1, an essential enzyme facilitating negative regulation of the “STING-dependent” antiviral response. In the recently published study, a Trex1 deficient model of AGS (Aicardi-Goutires Syndrome, an IFN associated autoimmune diseases linked to Trex1) was used to explain the development of human autoimmune diseases. The research describes the role of IFN in immune reactions.
Dr. Stetson also explained that the study can be applied in the development of effective therapies for treating IFN related autoimmune diseases such as AGS and lupus erythematosus.