Positive preliminary results has been announced by PositiveID, a developer of medical devices and molecular diagnostic systems for diabetes management, from its biotransport research study related to the company’s GlucoChip with the Diabetes Research Institute (DRI) at the University of Miami.
 Glucochip for monitoring glucose transport in diabetes patient
Glucochip for monitoring glucose transport in diabetes patient
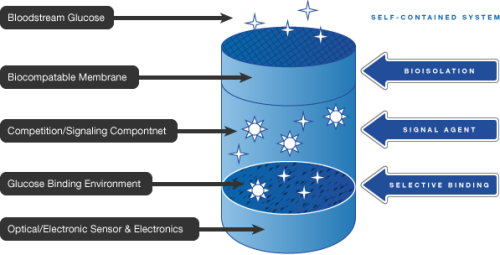
The company in now developing the GlucoChip, a glucose-sensing implantable microchip for individuals with diabetes, in partnership with Receptors. The test results illustrate that glucose moves successfully from body fluids, which is ensured by the membrane materials to be used with GlucoChip.
The membrane materials will act as an interface between the in vivo environment and the glucose monitoring system. The company has conducted the first animal studies to test the membrane materials by assessing the potential of glucose to reach the monitoring system by passing through the membrane.
A prototype of a stable, repeatable, continuous, closed-cycle glucose-monitoring system, which is the GlucoChip’s mission-critical component, has been developed by RECEPTORS and PositiveID. The continued positive results from the biotransport and biocompatibility study will make PositiveID incorporate a micro-electromechanical system, signal transduction system and the electronics of a radio frequency identification microchip into the glucose-monitoring system to complete the development of the GlucoChip.
The GlucoChip is developed on the basis of the company’s patent no. 7,125,382 for an embedded bio-sensor system. This patent includes a bio-sensor unit that employs the radio frequency identification technology and has a remote transponder along with a passively powered, implantable on-chip transponder in wireless communication. Currently, the company has five patents and patents pending surrounding the GlucoChip’s development.