Jun 9 2010
The Food and Drug Administration (FDA) of the US has approved hexaminolevulinate HCl (Cysview) technique for detecting the bladder’s non-muscle-invasive papillary cancer for patients with suspected or bladder cancer. This announcement was made by GE Healthcare, a subdivision of General Electric Company.
Cysview, an optical imaging agent, has been specified for utilization in the cystoscopic sensing of non-muscle-invasive type of papillary cancer in the bladder for patients known or suspected to have lesion(s) seen in earlier cystoscopy. Cysview is utilized along with the Karl Storz D-Light C type of Photodynamic Diagnostic (PDD) system for performing cystoscopy under the blue light setting on mode 2 as an appendage to the white light setting in mode 1.
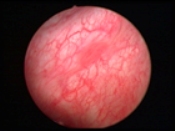 GE Healthcare View of bladder with white light crystoscope
GE Healthcare View of bladder with white light crystoscope
The problems experienced while diagnosing bladder cancer and its high recurrence rate has resulted in Cysview cystoscopy’s development. The optical agent Cysview is injected through a catheter into the bladder. Cysview accrues differentially in malignant cells. When malignant cells are illuminated with cystoscope’s blue, light red fluorescence is emitted by the cancerous lesions, highlighting the malignant areas.
Bladder cancer is the eighth most common cancer in women and the fourth most common in men, with smoking the most likely reason for bladder cancer. Difficult to detect, this cancer is initially indicated through red-colored urine and requires urine cytology and cystoscopy. The National Cancer Institute has reported that in the US number of people diagnosed with bladder cancer in 2009 exceeded 70,000, with an estimated 14,000 persons dying due the disease.
The standard procedure for diagnosing bladder cancer integrates white light cystoscopy and cytology. The non-invasive cytology technique detects cancer cells by using a sample of urine. This technique offers sensitivity and specificity while detecting high-grade lesions. It, however, does not offer information related to the extent and location of the disease. In case the test is positive for the cells of cancer, the next move involves using white light cystoscopy for direct visual inspection of mucosa and urothelium and for localizing the tumors. During this process the physician will also be able to conduct a transurethral resection of suspicious bladder regions. In the end, sample testing will indicate whether it is malignant.
GE Healthcare has licensed Cysview from a Norwegian pharmaceutical company Photocure ASA that creates and sells medical devices and pharmaceuticals for the photodynamic diagnosis and treatment of cancer and certain dermatology indications.