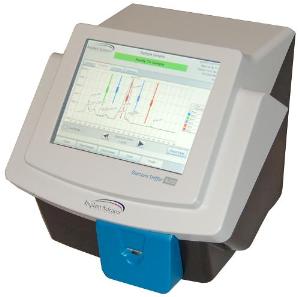
Implant Sciences Corporation, a high technology supplier of systems and sensors for homeland security and defense markets, announced today that its TSA-approved QS-B220 desktop explosives trace detector (ETD) has been certified by Service Technique de l'Aviation Civile (STAC) in France.
The QS-B220 is now certified for purchase and deployment to screen passengers, cargo, cabins, and baggage for explosives in airports throughout France. This marks the second major regulatory success for the QS-B220 following its approval by the U.S. Transportation Security Administration (TSA) for air cargo screening in January 2013.
 Implant Sciences Earns STAC Certification for QS-B220 Explosives Trace Detector (PRNewsFoto/Implant Sciences)
Implant Sciences Earns STAC Certification for QS-B220 Explosives Trace Detector (PRNewsFoto/Implant Sciences)
"This is a very significant achievement and milestone for Implant Sciences. STAC is a highly respected certification organization, and becoming certified opens the large French aviation market to us. A number of other governments including French territories, several European Union member nations, and a select number of countries around the world look to STAC certification in their purchasing decisions," stated Implant Sciences' President and CEO, Glenn Bolduc. "This is another step in our journey towards increasing our global market share and further establishing Implant Sciences as one of the major ETD manufacturers in the world."
"As a STAC-certified ETD with a non-radioactive source, the QS-B220 proves again that Implant Sciences is the new standard in trace detection," commented Darryl Jones, Implant Sciences' Vice President of Sales and Marketing. "Regulations on equipment containing radioactive materials are much stricter in France and other European nations than in the U.S., and we believe that there is a significant market opportunity for us with airports and other security customers that may have been reluctant to purchase competing ETD devices with a radioactive ion source."
About the QS-B220 Desktop Explosives Trace Detector
The QS-B220 uses Ion Mobility Spectrometry (IMS) to rapidly detect and identify trace amounts of a wide variety of military, commercial, and homemade explosives. With significantly lower maintenance requirements than competing systems, the QS-B220 can be deployed for a much lower total cost of ownership than other approved products. Featuring a radioactive material-free design, push-button maintenance and diagnostics, and a patented inCal™ internal automatic calibration system, the QS-B220 brings new levels of performance and convenience to desktop trace detection users with unsurpassed ease of use.
About Implant Sciences
Implant Sciences is the leader in next generation Explosives Trace Detection (ETD) technology. In January 2013, the Company became only the third ETD manufacturer, and the sole American-owned company, to currently have product approval from the US Transportation Security Administration. Implant Sciences has developed proprietary technologies used in its commercial explosives and drugs trace detection systems, which ship to a growing number of locations domestically and internationally. Implant Sciences' QS-H150 handheld explosives trace detector has received Qualified Anti-Terrorism Technology Designation and, in addition to receiving TSA approval for air cargo screening, the Company's QS-B220 has also received a Developmental Testing & Evaluation (DT&E) Designation by the U.S. Department of Homeland Security under the Support Anti-terrorism by Fostering Effective Technology Act of 2002 (the SAFETY Act). For further details on the Company and its products, please visit the Company's website.