Fluorescent sensors are employed to label and image a broad range of molecules. They provide an exclusive look within living cells. However, they can only really be employed in cells that have grown in tissues close to the surface of the body or in a lab dish, as their signal is lost when they are deeply embedded.
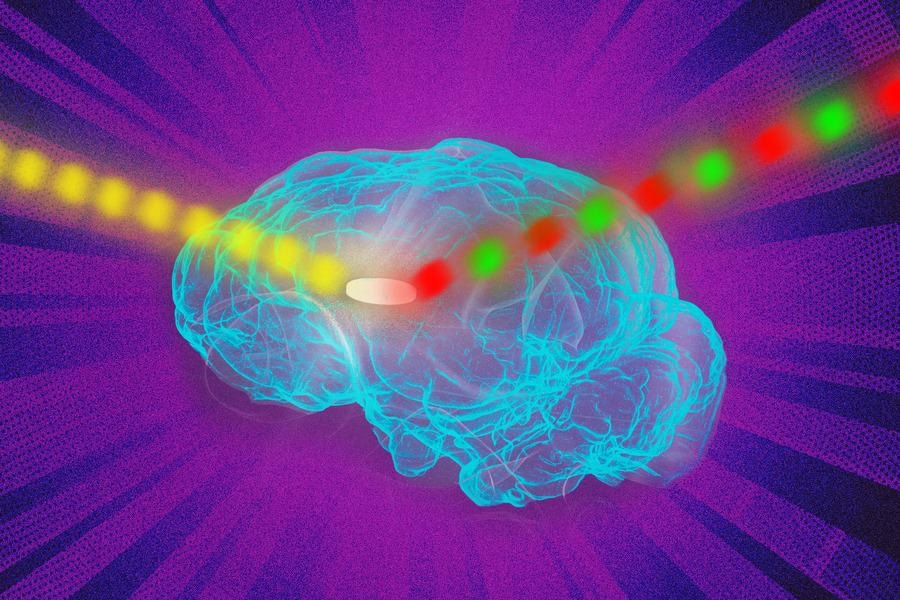 MIT engineers found a way to dramatically improve the signal emitted by fluorescing nanosensors. The researchers showed they could implant sensors as deep as 5.5 centimeters in tissue and still get a strong signal. Image Credit: Courtesy of the researchers and edited by MIT News.
MIT engineers found a way to dramatically improve the signal emitted by fluorescing nanosensors. The researchers showed they could implant sensors as deep as 5.5 centimeters in tissue and still get a strong signal. Image Credit: Courtesy of the researchers and edited by MIT News.
MIT engineers have currently developed a method to surpass that limitation. Using a unique photonic method they created for stimulating any fluorescent sensor, they could significantly enhance the fluorescent signal. With this technique, the scientists demonstrated they could embed sensors as deep as 5.5 cm in tissue and still receive a powerful signal.
This type of technology could allow fluorescent sensors to be used to monitor particular molecules within the brain or other tissues deep inside the body, for medical diagnosis or tracking drug effects, the scientists explain.
If you have a fluorescent sensor that can probe biochemical information in cell culture, or in thin tissue layers, this technology allows you to translate all of those fluorescent dyes and probes into thick tissue.
Volodymyr Koman, Research Scientist and Study Lead Author, MIT
Naveed Bakh SM ’15, Ph.D. ’20 is one of the lead authors of the study, which has been published in the journal Nature Nanotechnology. Michael Strano, the Carbon P. Dubbs Professor of Chemical Engineering at MIT, is the study's senior author.
Enhanced Fluorescence
Researchers employ a range of diverse fluorescent sensors, including carbon nanotubes, quantum dots, and fluorescent proteins, to label molecules within cells. These sensors’ fluorescence can be viewed by irradiating laser light on them. However, this is not possible in thick, dense tissue, or deep within tissue, as the tissue itself also discharges some fluorescent light. This light, known as autofluorescence, masks the signal emanating from the sensor.
All tissues autofluoresce, and this becomes a limiting factor. As the signal from the sensor becomes weaker and weaker, it becomes overtaken by the tissue autofluorescence.
Volodymyr Koman, Research Scientist and Study Lead Author, MIT
To surpass this restriction, the MIT team developed a method to control the frequency of the fluorescent light produced by the sensor so that it can be more effortlessly differentiated from the tissue autofluorescence. Their method, which they refer to as wavelength-prompted frequency filtering (WIFF), employs three lasers to produce a laser beam possessing an oscillating wavelength.
When this oscillating beam is irradiated on the sensor, it makes the fluorescence discharged by the sensor double its frequency. This enables the fluorescent signal to be effortlessly derived from the surrounding autofluorescence. Using this arrangement, the scientists were able to improve the signal-to-noise ratio of the sensors by over 50-fold.
One potential application for this type of sensing is to track the efficacy of chemotherapy drugs. To illustrate this potential, the team concentrated on glioblastoma, an aggressive type of brain tumor. Patients with this type of cancer typically go through surgery to eradicate as much of the tumor as possible, then are administered the chemotherapy drug called temozolomide (TMZ) to try to destroy any residual cancer cells.
This drug can have harmful side effects and it is not suitable for all patients, so it would be useful to have a way to track whether it is working or not in a straightforward manner, Strano states.
We are working on technology to make small sensors that could be implanted near the tumor itself, which can give an indication of how much drug is arriving at the tumor and whether it’s being metabolized. You could place a sensor near the tumor and verify from outside the body the efficacy of the drug in the actual tumor environment.
Michael Strano, Senior Study Author and Carbon P. Dubbs Professor of Chemical Engineering, MIT
When temozolomide is administered into the body, it gets split into smaller compounds, including one called AIC. The MIT researchers engineered a sensor that could identify AIC and demonstrated that they could insert it as deep as 5.5 cm inside an animal brain. They could read the sensor’s signal even through the skull of the animal.
These types of sensors could also be built to spot molecular signatures of tumor cell death, like reaction oxygen species.
“Any Wavelength”
Besides identifying TMZ activity, the scientists showed that they could use WIFF to improve the signal from a range of other sensors, including carbon-nanotube-based sensors that Strano’s lab has earlier built to identify riboflavin, hydrogen peroxide, and ascorbic acid.
The technique works at any wavelength, and it can be used for any fluorescent sensor. Because you have so much more signal now, you can implant a sensor at depths into tissue that were not possible before.
Michael Strano, Senior Study Author and Carbon P. Dubbs Professor of Chemical Engineering, MIT
For this current research, the scientists employed three lasers together to form the oscillating laser beam, but in future research, they want to employ a tunable laser to form the signal and enhance the method even more. This should become more viable as the price of tunable lasers drops and they become faster, the scientists state.
To make fluorescent sensors simpler to use in human patients, the scientists are designing sensors that are biologically resorbable, so they would not have to be surgically taken out.
The study received funding from the Koch Institute for Integrative Cancer Research and Dana-Farber/Harvard Cancer Center Bridge Project. Further funding was offered by the Swiss National Science Foundation, the Israeli Science Foundation, the Japan Society for the Promotion of Science, the Zuckerman STEM Leadership Program, the King Abdullah University of Science and Technology, and the Arnold and Mabel Beckman Foundation.
Journal Reference:
Koman, V. B., et al. (2022) A wavelength-induced frequency filtering method for fluorescent nanosensors in vivo. Nature Nanotechnology. doi.org/10.1038/s41565-022-01136-x.